Getting a colonoscopy?
Consider a
different
way to prep

Why do I need a colonoscopy?
Getting regular colon cancer screenings starting at age 45 can help to prevent and detect colon cancer and other gastrointestinal issues.
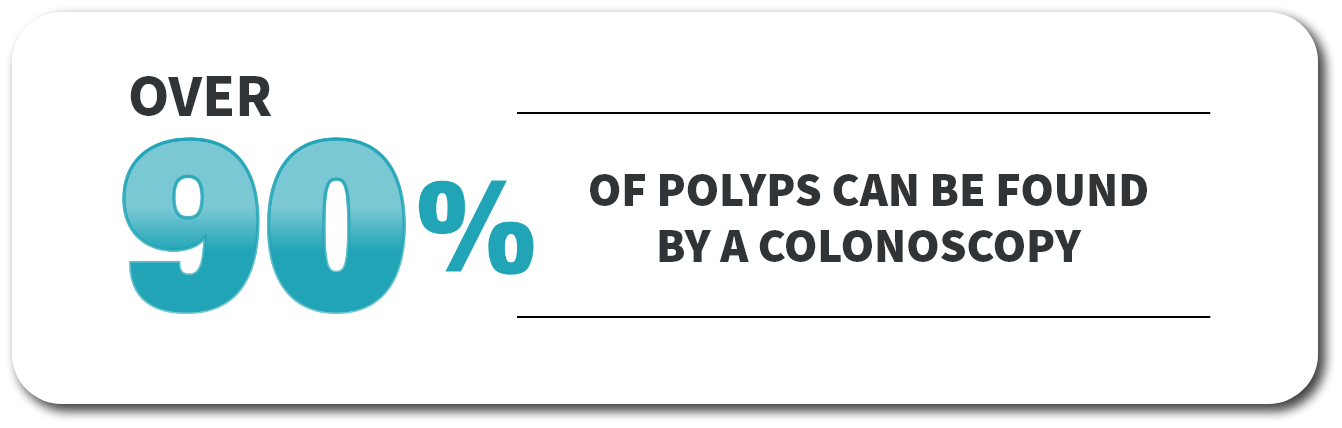
Over
OF POLYPS CAN BE FOUND BY A COLONOSCOPY
A colonoscopy is a common painless procedure during which your healthcare provider—usually a gastroenterologist—uses a camera called a colonoscope to look inside your rectum and entire colon for small growths called polyps. If not removed, polyps may become cancerous. Having a clean colon prior to your procedure is the key to a successful colonoscopy.
GET MORE BOWEL PREP INFOYour PLENVU®
dosing guide
See instructions and tips on how to take each dose of your PLENVU® bowel prep.
Get STartedWhy PLENVU®?
How you prep for your colonoscopy can make a difference
Bowel prep has made great strides over the years. PLENVU® offers a quality prep medicine with less to drink and 2 dosing flavors.
PLENVU® was designed with you in mind:
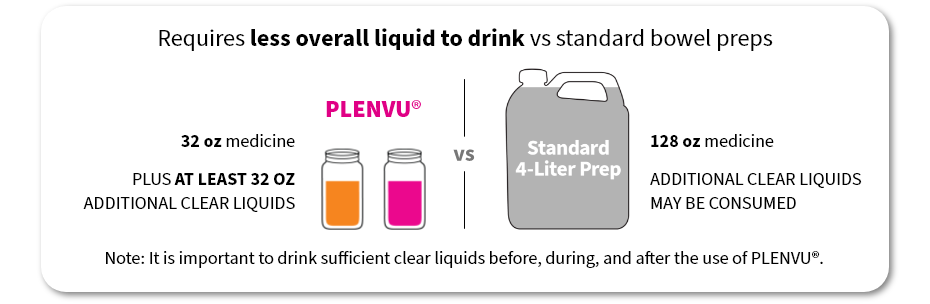
Requires less overall liquid to drink vs standard bowel preps
PLENVU®

32 oz medicine
Plus AT LEAST 32 OZ ADDITIONAL CLEAR LIQUIDS


128 oz medicine
ADDITIONAL CLEAR LIQUIDS may be consumed
Note: It is important to drink sufficient clear liquids before, during, and after the use of PLENVU®.
TWICE AS MANY PEOPLE FOUND THE TASTE OF PLENVU® “VERY ACCEPTABLE”
compared to those who took another leading bowel prep.*,†
*Based on diary ratings given by patients who took PLENVU® or Suprep® during a clinical trial. There were no significant differences between the ratings given for easy to follow instructions, easy to drink, effectiveness of bowel cleansing, and interference with normal daily activities
†Very acceptable: 15.2%, PLENVU® vs 7.5%, Suprep
Hear what these patients said about PLENVU®
Learn more about PLENVU® from these patients who share their personal bowel prep experiences.
“I would highly recommend to people who are getting a colonoscopy to talk to their doctor about PLENVU®.”
– Danielle, real PLENVU® patient
“I liked that PLENVU® was easy to prepare. I was able to mix the powdered contents really well, which made it smooth and easy to drink.”
– Noel, real PLENVU® patient
Additional support is available to help you have a successful colon cleanse
EXPLORE PLENVU® RESOURCESINDICATION
PLENVU® (polyethylene glycol 3350, sodium ascorbate, sodium sulfate, ascorbic acid, sodium chloride, and potassium chloride
for oral solution) is a prescription medication used by adults to clean the colon before a colonoscopy.
for oral solution) is a prescription medication used by adults to clean the colon before a colonoscopy.
IMPORTANT SAFETY INFORMATION
- Do not take PLENVU® if you have a blockage in your intestine (bowel obstruction), an opening in the wall of your stomach or intestine (bowel perforation), problems with food or fluid emptying from your stomach (gastric retention), a problem with food moving too
INDICATION
PLENVU® (polyethylene glycol 3350, sodium ascorbate, sodium sulfate, ascorbic acid, sodium chloride, and potassium chloride for oral solution) is a prescription medication used by adults to clean the colon before a colonoscopy.
IMPORTANT SAFETY INFORMATION
- Do not take PLENVU® if you have a blockage in your intestine (bowel obstruction), an opening in the wall of your stomach or intestine (bowel perforation), problems with food or fluid emptying from your stomach (gastric retention), a problem with food moving too slowly through your intestines (ileus), a very dilated large intestine, or an allergy to any of the ingredients in PLENVU®.
- PLENVU® and other bowel preparations can cause serious side effects including loss of body fluid (dehydration) and changes in blood salts (electrolytes) in your blood. These changes can cause abnormal heartbeats that may result in death, seizures (even if you have never had a seizure), or kidney problems. Your chance of having fluid loss and changes in body salts with PLENVU® is higher if you have heart problems, kidney problems, or take water pills, high blood pressure medicine, or non-steroidal anti-inflammatory drugs (NSAIDS).
- Your healthcare provider may do blood tests after you take PLENVU® to check your blood for changes. Tell your healthcare provider right away if you have any symptoms of too much fluid loss (dehydration) including vomiting, dizziness, heart problems, kidney problems, seizures, dry mouth, urinating less often than normal; headache, or feel faint, weak, or lightheaded, especially when you stand up.
- PLENVU® can cause ulcers of the bowel or bowel problems (ischemic colitis). Tell your healthcare provider right away if you have severe stomach-area (abdomen) pain or rectal bleeding.
- PLENVU® can cause serious allergic reactions that may include skin rash, itching, raised red patches on your skin (hives); swelling of the face, lips, tongue, and throat; and kidney problems.
- The most common side effects in patients taking PLENVU® were nausea, vomiting, dehydration, and stomach pain or discomfort.
- Tell your healthcare provider about all of your medical conditions and medicines you take, including prescription, nonprescription medicines, vitamins, and herbal supplements before you take PLENVU®.
These are not all the possible side effects of PLENVU®. Ask your healthcare provider for more information.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch
or call 1-800-FDA-1088.
or call 1-800-FDA-1088.
For product information, adverse event reports, and product complaint reports, please contact:
Please click here for full Prescribing Information, including Medication Guide and Instructions for Use.